The 2018 Nobel Prize in Physiology or Medicine was awarded jointly to James Allison of MD Anderson Cancer Center at the University of Texas at Houston and Tasuku Honjo of Kyoto University for work that “established an entirely new principle of cancer therapy.” Each independently discovered that our immune system is restrained from attacking tumors by molecules that function as “brakes.” Releasing these brakes (or “brake receptors”) allows our body to powerfully combat cancer.
This remarkable advance has been a long time coming. The idea that our natural defenses could be mobilized against tumors extends back more than a century, to the case of a young woman named Elizabeth Dashiell. In 1890 Dashiell, then seventeen years old, consulted a Manhattan surgeon, William Coley, for a painful swollen hand. After several weeks of conservative measures, Coley operated on her hand and found her bones encased by a sarcoma—a cancer of the connective tissue. He sought to cure the cancer by amputating Dashiell’s arm just below her elbow, but tumors soon appeared in her neck and abdomen. Several months later she died at home, with Coley at her bedside.
Dashiell’s death had a profound emotional impact on the young surgeon. He began to comb through the records of New York Hospital, searching for cases of sarcoma with better outcomes. One success stood out from the expected fatalities: Fred Stein, an immigrant housepainter, had undergone multiple operations for a sarcoma that had been rapidly spreading in his neck. His case was labeled “absolutely hopeless” by his surgeon. Then Stein developed a bacterial infection in the region of the tumor. Antibiotics didn’t exist at the time, but his own white blood cells were able to eradicate the bacteria. As they did, the cancer shrank and ultimately disappeared.
This stunning outcome prompted Coley to search for a way to make the body combat tumors, as Stein’s apparently did. The surgeon initially inoculated cancer patients with what he called “laudable pus,” extracts of bacterial abscesses, and then with bacteria themselves. While Coley documented occasional instances of tumors shrinking, he never arrived at a reproducible way to cure cancer by stimulating a patient’s natural defenses.1
Over the ensuing decades, cancer treatment largely consisted of surgery to excise tumors, radiation to burn them, or chemotherapy to poison them. There were moments, though, when it seemed that Coley’s dream would be realized. Researchers studying the immune system discovered the molecules interferon and interleukin-2; each was initially hailed as a promising treatment based on their dramatic effects in shrinking tumors in rodents. But both failed to have robust and broad benefits when used to treat cancer in humans.
Such frustrating setbacks did not deter James Allison, a young scientist from the small town of Alice, Texas. Counseled by mentors to stay away from studying the relationship between the immune system and cancer, which they claimed would be a dead end, Allison rejected their advice.2 He was intrigued by a molecule called CTLA-4 that was thought to stimulate immune cells, but he came to an opposite view: CTLA-4 was a brake rather than an accelerator. In an elegant series of experiments in mice, he showed that releasing the CTLA-4 brake allowed the rodent’s immune system to attack and eradicate tumors. Working independently in Japan, Tasuku Honjo identified a different molecule, PD-1, and proved that it too was a brake on immune cells; once PD-1 was counteracted, the rodent immune system was freed to combat cancers.
In 2004 the first human trials of agents that released these brakes were conducted, and were initially declared failures. When patients receive radiation or chemotherapy, the treatment is judged successful if tumors shrink by at least 50 percent in diameter; this shrinkage occurs within weeks to a few months. Patients with metastatic melanoma who received blockers of CTLA-4 had no significant shrinkage after months. The failure seemed to be another example of the differences between a rodent’s immune system and a human’s. Pfizer, one of the companies producing a potential blocker, then abandoned it.
But clinicians conducting another apparently failed trial noticed that many months after treatment with the blockers was suspended, the tumors either stopped growing or began to shrink. Instead of assessing efficacy in the short term, as was usual for radiation and chemotherapy, the researchers measured patient survival over a period of years. In 2010 the study results were presented at a major cancer meeting: a quarter of the patients treated with blockers for widespread melanoma were alive after two years; their predicted survival had been a mere seven months.
One of the most stunning successes of this treatment is the case of President Jimmy Carter. In the summer of 2015 he was diagnosed with melanoma that had spread to his liver and brain. With standard radiation and chemotherapy his prognosis was dismal, measured in weeks to a few months. Carter received a new PD-1 blocker and remains in remission nearly four years later. Metastatic melanoma has proved to be one among several previously intractable cancers that has yielded to immune therapy. Clinical trials in lung cancer, Hodgkins lymphoma, bladder cancer, Merkel cell carcinoma, and others have shown dramatic remissions and raised the prospect of some patients being cured. In general, a quarter to a third of treated patients react positively.
Advertisement
The advent of successful immune therapy for cancer comes with a price. It often causes toxic side effects, as the unleashed immune system attacks not only the tumor but normal tissues as well. Patients can suffer intense inflammation of the bowels, skin, and thyroid and adrenal glands. Then there is the cost of the treatments, typically more than $100,000 per year.
The work of Allison and Honjo inspired Matt Richtel to write An Elegant Defense. A Pulitzer Prize–winning journalist at The New York Times, he “picked up [his] pen,” hoping for a happy ending, that the new immune therapy would save the life of his best buddy from childhood, Jason Greenstein, who had advanced Hodgkin’s lymphoma. In addition to Greenstein, Richtel introduces the reader to Merredith Branscombe, a businesswoman with lupus; Bob Hoff, a former government lawyer with HIV whose immune system suppresses the virus; and Linda Segre, a golfer with rheumatoid arthritis. Each case poses a fundamental question in immunology. Why doesn’t Greenstein’s body recognize his lymphoma as dangerous and fight it? What causes Branscombe’s and Segre’s immune systems to damage their healthy tissues? How does Hoff naturally contain a pathogen like HIV without the need for antiviral drugs?
To address these questions, Richtel first explicates for the lay reader the intricate biology of our immune system. He explains how important immune cells—T cells, macrophages, and dendritic cells—distinguish endogenous entities from foreign ones, and how pathogens trigger the immune cells into a defensive response. He also explains how our antibodies are made by B cells, a process involving the reshuffling of DNA, and highlights recent findings on the microbiome, the bacteria in our gut that coexist with our immune system.
He succeeds in this formidable task using colloquial prose with touches of humor. While the word “immunity” connotes defense, Richtel writes:
The war metaphor is misleading, incomplete—even arguably dead wrong. Your immune system isn’t a war machine. It’s a peacekeeping force that more than anything else seeks to create harmony…. This is not just because we don’t want to hurt our own tissue. It is also because we need many of the alien organisms that live on and in us, including the billions of bacteria that live in our guts….
But what specifically does the microbiome help with?
Digestion, nutrition, obesity—broadly, how much energy we take from foods and how effectively we squeeze nutrients from them.
These commensal microbes, Richtel continues, do not threaten us but serve as essential allies. By unnecessarily eradicating them with excessive use of antibiotics or antibacterial soaps, “we risk impairing bacteria that contribute to the effectiveness of our immune system’s function.” This is the so-called hygiene hypothesis, which argues that excessive efforts at cleanliness may, ironically, weaken our defenses and increase our risk for allergic and autoimmune disorders.
In contrast to the bacteria of the microbiome are pathogenic microbes. When they try to invade us,
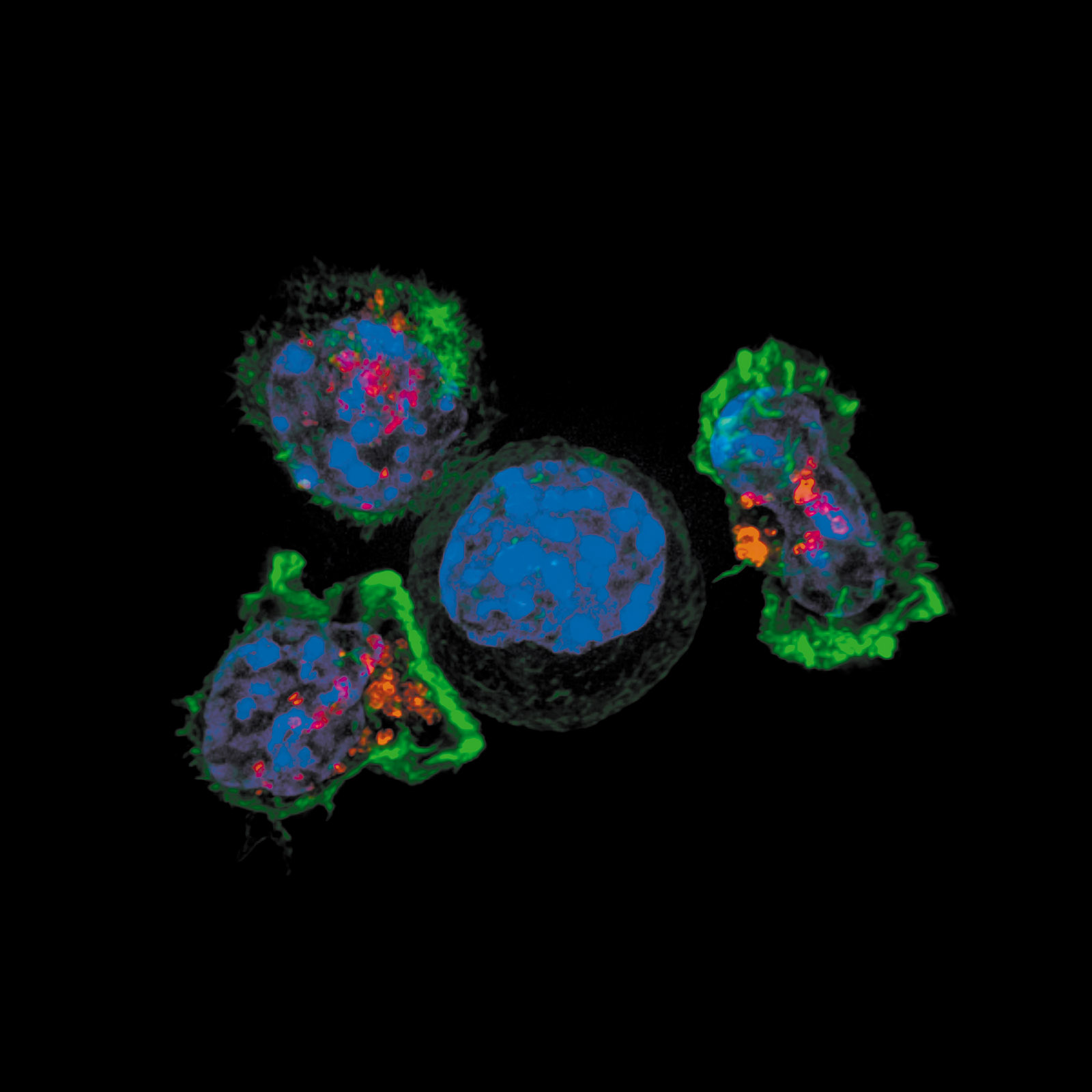
immune cells show up in force and devour the infection. Some immune cells blow themselves up in the process. Others nip off parts of the infection and carry them away to be assessed in a defense hub called a lymph node. There, the bits of infection are shared with swarms of passing defenders called T cells and B cells. These are the immune system’s most advanced fighters; they are, in fact, two of the most effective biological structures in the world. What makes T cells and B cells so remarkable is that they are extremely specific. Each one of the billions of them in your body is tailored through a quirk of genetics to recognize a very specific infection. Once a T cell or B cell finds its evil mate, its infection doppelgänger, it can set in motion a powerful defense, following hard on the innate reaction, bringing defenders trained specifically to bounce out this particular antigen.
The conundrum posed by cancer—why can tumors with characteristics distinct from healthy tissues evade the immune system’s surveillance?—is answered by Richtel, who draws on Honjo’s and Allison’s research:
The last few decades of immunology…have taught us [that] the immune system…can be duped. Sometimes a disease takes root and starts to grow and spread and then tricks the immune system into thinking it isn’t so bad after all. It deceives the entire defense system into helping it grow. This is what happened to Jason.
Delving deeply into this phenomenon, Richtel interviews oncologists who elaborate that cancer cells can look like healthy ones:
Advertisement
Part of the way that Hodgkin’s and other cancers disguise themselves is by tricking the T cells that would ordinarily help kill off the mutation. What the cancer does is send a signal to the T cell to self-destruct…. At the same time, now that the immune system had received a message that the cancer was “self” and not alien, the immune system actually set out to protect and support the cancer…. The tumor co-opts the immune system and says, “I’m okay. I just want you to help me grow.”
The patient stories in An Elegant Defense are vividly told. The impact of lupus on Merredith Branscombe is painfully apparent: “Merredith’s immune system had turned on her own body as if it were itself an alien threat…. There were middle-of-the-night emergency room visits with inflammation around her heart, blood in her stool, and pain ‘like someone had plunged knives into both sides of my body and were just…turning and driving those knives deeper and deeper into my muscles.’” Like some patients with lupus, Merredith manifests photosensitivity:
She pulled her black shirt over her left hand, protecting it from the sun. She held her right hand out in front of me, palm down…. The uncovered hand began to swell. It turned red…. She withdrew her left hand from her shirt, and put the pair of them side by side. Now it was more glaring, her left hand white and a touch puffy, which reflected the regular inflammation, her right hand red and visibly swollen.
“My immune system,” she said, “is always attacking me.”
Linda Segre’s autoimmune disorder, rheumatoid arthritis, is marked by profound pain and swelling with destruction of her joints. Of the genesis of her malady, Richtel writes:
The clues to and the catalyst of her illness were actually there all along to be discovered if given proper scrutiny. In addition to her family history, she suffered extreme stress, sleeplessness, and a case of strep throat that might have kicked her immune system into overdrive.
This list of possible causes should come with a prominent caveat, as the reason why most people develop autoimmune disorders is still obscure. As for Bob Hoff, despite intensive study of his immune system at the NIH, precisely how his body prevents HIV from behaving in its typical destructive way remains a mystery.
While successfully communicating the science of Allison and Honjo and related clinical advances to a lay reader, Richtel occasionally lapses into hyperbole: he calls Greenstein “one of the first fifty patients to try one of the greatest developments in the history of medicine…. He stood at the very edge of human achievement as modern science challenged one of the most enduring and effective killing techniques in the pantheon of disease.” Richtel places our new understanding of the immune system “on par with the greatest human achievements” and quotes an immunologist at UCLA asserting that recent discoveries are “as significant as the discovery of antibiotics.”
His enthusiasm reflects that of other writers on the immune system. Books on the subject are often marketed with the promise that we can fine-tune our defenses by adopting a specific lifestyle, such as daily meditation, a diet of “anti-inflammatory” foods, and precisely measured hours of sleep. Daniel Davis, a professor of immunology at the University of Manchester in the UK, is refreshingly sober in assessing such popular notions in The Beautiful Cure:
All kinds of stresses have been linked with diminished immune responses, from burnout at work to unemployment…. Well over a hundred clinical studies have reported that stress can contribute to poor health, which leads many to suppose that a super-charged lifestyle perhaps increases our risk of all kinds of illnesses, from autoimmune disease to cancer. The topic remains controversial, however, because so many factors affect our ability to fight disease that it is difficult to assess the effect of any one.
Rather than accept stress as a distinct cause of immune system impairment, Davis rightly cautions us not to “explore the relationship between stress and health, without the added complication of stressed individuals being more likely to exercise less, sleep poorly, drink alcohol or smoke.” The same caveats hold for the alleged salubrious effects on immunity of mindfulness and exercise: “There is good evidence that t’ai chi can help improve pain and physical mobility for elderly arthritis patients. Whether or not t’ai chi impacts the immune system, however, is controversial.”
Davis emphasizes that most published reports on the effects of lifestyle changes on the immune system are methodologically flawed: they involve small numbers of subjects, not selected randomly and without an appropriate control group. Davis quotes from a review of sixteen clinical trials: “Because of methodological flaws in existing studies, further vigorously designed large-scale placebo-controlled, randomized trials are needed.” He warns against “exaggerating the positive effects of mindfulness meditation on immune system dynamics until these effects are further replicated and additional studies are performed.”
While Davis enthusiastically applauds the landmark discoveries of Allison and Honjo, he also offers an intelligent and insightful analysis of the field’s unknowns:
This is still only a beginning. We now know of over twenty other brake receptors in the immune system. Most of these switch off specific types of immune cell: Natural Killer cells, macrophages, dendritic cells, T cells, B cells or others. We must now test, in academic labs and companies large and small, whether or not antibodies that block these receptors, individually or in combination, will unleash immune cells to tackle different types of cancer.
Furthermore, Davis writes that we are unable to predict which types of cancer will be most affected by releasing the brakes on a particular type of immune cell. The system, he avers, “is too complex and our understanding too slight.”
In that regard, there is a pressing need to “personalize” the use of current CTLA-4 and PD-1 blockers, given their toxic side effects. The goal is to select patients most likely to benefit and spare those who won’t. Davis imagines identifying
which brake receptors are present at the surface of a person’s immune cells. This would allow us to select a[n]…inhibitor that targets those particular receptors. A person’s tumour can also be analysed to determine whether or not it contains the protein molecules that trigger particular brake receptors on immune cells. This could, in principle, predict whether or not blocking the PD-1 brake system, for example, is likely to benefit a patient.
As straightforward as this seems, Davis states that “this has not proved to be so easy.” He explains:
First, the brakes are dynamic; knowing what is keeping the immune system in check one day might not reflect the situation the next day. Also, things can change as a result of treatment; as one brake comes off thanks to a[n]…inhibitor, a tumour may adapt to exploit another brake system.
In addition, there is biological variability among cancer cells even within an individual patient:
A single tumour is sometimes said to be not a single disease but over a million different ones, with each of its millions of cancer cells being slightly different…. We don’t yet know which molecules on a person’s cancer are the best to target, we don’t yet know whether or not every cancer cell would have to possess the same signature, we don’t yet know how to limit the possibility of healthy bystander cells being attacked, causing unwanted side effects.
Importantly, Davis does not ignore the economics of these novel immune therapies:
It would be deceptive—dishonest—to write about new medicines without mentioning the financial problems that stand in our way: we sorely need new international institutes and different ways of funding medical research and medicines, where the well-being of humanity, and other life on earth, is paramount and financial profit irrelevant. I hope this is the brave new world that awaits us.
This is an honorable hope but realistically elusive, as societies struggle to incentivize the high-risk endeavor of developing breakthrough treatments, with their high rate of failure, while containing unregulated greed when success occurs.
The research honored by the 2018 Nobel Prize is a historic advance that already has restored the lives of many with cancer. But sadly, Davis notes, there are still large gaps in our knowledge of how to optimally unleash the immune system. After treatment with a PD-1 blocker, Jason Greenstein’s lymphoma shrank, but then rapidly grew again, ending his life.