In news coverage of the unfolding disaster at Japan’s Fukushima Daiichi nuclear plant, two themes have been particularly prominent. One is the paramount problem of potentially lethal radiation being released from the stricken reactors to a large area of Japan and beyond. Even now, new reports of radiation continue to surface. (As I write this from Colorado it was just announced that a tiny amount of radioactive detritus from Japan has arrived here.) The second—with implications for nuclear facilities around the world—is the vulnerable design of the plant’s six reactors and their storage pools for nuclear waste, all of which were at risk of losing their cooling water and could have caught fire in the days after the earthquake. Often missing in this discussion, however, is an analysis of the particular kind of radiation that has been detected and what it may reveal about the accident.
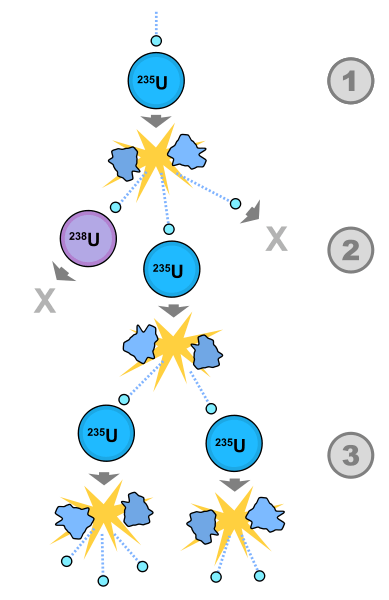
A key, and fortunate, development over the past few days has been the discovery that the radioactivity that has traveled as far as Tokyo—and Colorado for that matter—mainly consists of an isotope of iodine-131. This may seem like an odd bit of good news but I will explain. First we need to know how iodine-131 is produced. In a reactor it is the uranium-235—usually 3 or 4 percent of the total uranium, contained in “fuel rods” that are inserted into the reactor—that produces the nuclear fission reaction. This is how it works: if you think of the uranium-235 nucleus as a liquid drop, then when the drop is struck by a neutron it begins to agitate and if the agitation is sufficiently strong it reaches the right energy and splits into droplets—what are known as the fission fragments. In addition, more neutrons are produced and these cause further fission and hence the nuclear “chain reaction.” The masses of these fission fragments—which consist of unstable nuclei in the middle of the periodic table, plus the emitted neutrons, somewhat less than three on average—are less than the mass of the uranium-235 and the initial neutron. The difference in mass produces an energy which is given by Einstein’s formula E=mc2. This is the energy of fission.
The reaction we want to focus on is the one that produces iodine-131 along with an isotope of the rare earth element yttrium. This isotope of iodine is unstable and has a half-life of about 8 days. If one ingests iodine-131 in modest quantity the electrons can cause thyroid cancer by cellular mutation. (Interestingly a larger dose can kill these cancerous cells.) So why on earth is it good news that this isotope was found near the Fukushima Daiichi reactors? Here we must enter into the matter of the storage pools for nuclear waste.
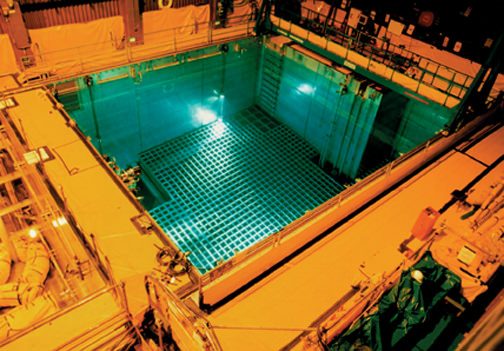
When these reactors work they produce large amounts of nuclear waste in the form of spent fuel. The spent fuel rods contain both depleted uranium—uranium that through the fission reaction has been depleted of its uranium-235 content—and the highly radioactive fission fragments of which there are a very substantial variety. When the nuclear industry began in the United States in the late 1950s it was decided to store this waste temporarily in holding pools, a model also used by the Japanese. The waste would be well below the surface of the water, which would act to cool the spent fuel rods to keep them from burning. It would also act as a shield for the radioactivity of the stored rods. Even though fission is no longer taking place in these rods, the radioactivity generates heat. I was once given the plutonium pit of a nuclear weapon to hold and it was warm. So long as these elements retain their radioactivity, which can be for many decades, heat is generated.
To date, the US has produced an estimated 70,000 metric tons of nuclear waste, and it was long assumed that more permanent storage for it would be established. But with continued resistance to building such a long-term facility at Yucca Mountain in Nevada, or anywhere else, there has been no progress and at the moment that are some 100 storage pools scattered around the country, next to the reactors that produced the waste they contain. After many decades, so much waste is stored in these pools that many are considered “dense packed,” having reached a point at which the density of the waste is comparable to that of the fuel in the core of the reactor.
A typical power reactor of this type contains about eighty metric tons of uranium in its core. Since the fuel rods are removed every few years, a storage pool in the United States might contain as much as 400 tons of spent fuel deriving from these exchanges of fuel. The pools at Fukushima Daiichi are about forty by forty feet with a depth of forty five feet. In principle the stored fuel rises to no more that fifteen feet above the bottom. These pools are less densely packed than their American counterparts; they contain some eighty tons of spent fuel. But they are directly above the reactors.
Advertisement
Here lies the problem that has become so urgent in the Japanese disaster. If there is a loss of water in the pools, the cladding of the fuel elements—the exterior of the fuel rods—can melt, resulting in the release of the radioactive fragments. In the Japanese reactors the pools lie within the containment structure of the reactor itself (the steel shell) and do not have separate containment structures. This means that if the containment structure of the reactor is breached—as it was in several of the reactors at the Fukushima Daiichi plant—radioactive elements from any melted fuel rods in the pools when they suffer a loss of water can escape and be dispersed into the surrounding environment.
The different radioactive elements pose different levels of risk. Besides iodine-131, I want to focus on another dangerous isotope, cesium-137. Like the iodine isotope, cesium-137 is unstable and decays into electrons, but with a much-longer half-life of about thirty years. It is a nasty cancer-causing stuff. Vast amounts of cesium-137 were released in the Chernobyl accident from the reactor core and because of its slow decay, an “exclusion zone” of some 1,100 square miles around the site will in theory be uninhabitable for perhaps two centuries. If this isotope had been released in large quantities in the Japanese accident it would have been an unmitigated disaster.
(Adding to our concerns, one of the Japanese reactors, Reactor 3, uses a different kind of fuel called MOX. This is uranium fuel with a small amount of plutonium mixed in. The fission of plutonium produces other radioactive isotopes, and plutonium itself—even in tiny quantities—is extremely dangerous to ingest. Reports about the condition of this reactor must be watched closely.)
What accounts, then, for the appearance of iodine-131 but not large quantities of cesium-137? The waste in these pools is stored for long periods of time. This means that the shorter lived isotopes such as iodine 131 decay away but the cesium-137 remains. Indeed there is about as much cesium-137 stored in the pools as there is in the core of the reactor. Now you see why this news about the iodine was good. It means that the radioactive emission from the reactors was coming from the reactors themselves and not the pools. (A small amount of cesium was released from the core of the reactor, but it has not been found in any quantity far from the plant and is negligible compared to the amount that could be emitted from the pools.) It is clear that putting water into these pools to cool them after the accident was touch and go. If that had failed it would have been a calamity. If this situation persists we may be looking at an accident less from the point of view of seriousness like Chernobyl and more like Three Mile Island. These two accidents were produced by human failures and not an act of God like an earthquake.
What are the lessons? There will be many but the most obvious to me is that we must find a better way of storing this waste than in the pools. As bad as the Japanese situation has been, we are lucky that the pools themselves do not seem to have emitted substantial radiation. But the disaster highlights the urgent and growing problem of nuclear waste storage all over the world. Some proposals, such as Yucca Mountain, have become political footballs, and seem unlikely to get anywhere. Others, including the use of “dry casks”—storing already cooled spent fuel in steel boxes that are pumped with inert gas and then sheathed in their own concrete and steel containers—are feasible and at least provide a better short-term answer. But they are expensive and the nuclear industry is resisting them. Nuclear waste storage is clearly an international problem and should have an international solution.
I am not against nuclear energy. Quite the contrary. But I do not want to see any further construction until the matter of the waste has been resolved.
This morning it was announced that small amounts of plutonium had been found in soil samples taken some 500 yards from the Fukushima reactors. I would like to put this in context. I thank my colleague Carey Sublette for very helpful remarks.
Advertisement
In the years between 1952 and 1963 some tons of plutonium were injected into the atmosphere in the course of above ground nuclear weapons testing. This has come back to earth as fallout, hence it would have been surprising if plutonium had not turned up in these Japanese soil samples. However what is significant is the isotopic content of the samples. In a nuclear weapon the predominant isotope is plutonium-239, which has a half life of some 24,000 years; other isotopes are also present in small amounts since they are also produced by the same reactors that produced the plutonium-239. But in the Japanese soil samples more of a different isotope, plutonium-238, was present than would be expected from such fallout.
Like all isotopes of plutonium, plutonium-238 is unstable and has a half life of some 86 years. It is a useful energy source and can be found in space probes. Curiously it was the first plutonium isotope to have been discovered in 1941. Its enhanced presence in the soil samples means that it comes from the reactors and was likely produced in the burn up of nuclear fuel. It is a useful tool for diagnosing the likely state of these spent fuel elements. It indicates that there must have been some breakage in their containment. There is no more danger from this plutonium than from the rest of the plutonium in the soil but as a diagnostic it should be watched carefully.